Publikationen - Research Articles, Reviews, Books
Forschungsgebiete
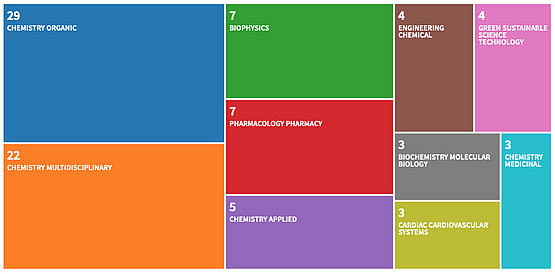
2016-2024
75. Bassetto Jr, C.A.Z., Pfeffermann, J., Yadav, R., Strassgschwandtner, S., Glasnov, T., Bezanilla, F., Pohl, P.
Photolipid excitation triggers depolarizing optocapacitive currents and action potentials
Nat. Commun. 2024, 15, in press
DOI: 10.1038/s41467-024-45403-y
74. Varbanov, H. P., Belaj, F., Glasnov, T., Herbert, S., Brumby, T., Mösch-Zanetti, N.C.
Neutral W(V) Complexes Featuring the W2O2(µ-O)2 Core and Amino Acids or EDTA Derivatives as Ligands: Synthesis and Structural Characterization
Inorganics. 2023, 11, 114
DOI: 10.3390/inorganics11030114
73. Varbanov, H. P., Glasnov, T., Belaj, F., Herbert, S., Brumby, T., Mösch-Zanetti, N.C.
New strategies towards advanced CT contrast agents. Development of neutral and monoanionic sulfur-bridged W(V) dimeric complexes
Dalton Trans. 2022, 29, 11086
DOI: 10.1039/D2DT01470J
72. Saitov, A., Kalutsky, M.A., Galimzyanov, T.R., Glasnov, T., Horner, A., Akimov, S.A., Pohl, P.
Determinants of lipid domain size
Int. J. Mol. Sci. 2022, 23, 3502
DOI: 10.3390/ijms23073502
71. Pfeffermann, J., Eicher, B., Boytsov, D., Hannesschläger, C., Galimzyanov, T.R., Glasnov, T.N., Pabst, G., Akimov S.A., Pohl, P.
Photoswitching of model ion channels in lipid bilayers
J. Photochem. Photob. B: Biology 2021, 224, 112320
DOI: 10.1016/j.jphotobiol.2021.112320
70. Saitov, A., Akimov S.A., Galimzyanov, T.R., Glasnov, T.N., Pohl, P.
Ordered lipid domains assemble via concerted recruitment of constituents from both membrane leaflets
Phys. Rev. Lett. 2020, 124, 108102
DOI: 10.1103/PhysRevLett.124.108102
69. Stadler, E., Tassoti, S., Lentes, P., Herges, R., Glasnov, T., Zangger, K., Gescheidt, G.
In situ observation of photoswitching by NMR spectroscopy: A photochemical analogue to the exchange spectroscopy experiment
Anal. Chem. 2019, 91, 11367-11373
DOI: 10.1021/acs.analchem.9b02613
68. Tiapko, O., Shresta, N., Lindinger, S., de la Cruz, G.G., Graziani, A., Klec, C., Butorac, C., Graier, W., Kubista, H., Freichel, M., Birnbaumer, L., Romanin, C., Glasnov, T.N., Groschner, K.
Lipid-independent control of endothelial and neuronal TRPC3 channels by light
Chem. Sci. 2019, 10, 2837-2842
DOI: 10.1039/C8SC05536J
67. Svobodova, B., Lichtenegger, M., Platzer, D., di Giuro, C.M.L., de la Cruz, G.G., Glasnov, T., Schreibmayer, W., Groschner, K.
A single point mutation in the TRPC3 lipid-recognition window generates supersensitivity to benzimidazole channel activators
Cell Calcium 2019, 79, 27-34
DOI: 10.1016/j.ceca.2019.02.007
66. Morozova, A.D., Muravyova, E.A., Shishkina, S.V., Sysoiev, D., Glasnov, T., Musatov, V.I., Desenko, S.M., Chebanov, V.A.
Features of 3-amino-5-methylisoxazole in heterocyclizations involving pyruvic acids
Chem. Heterocycl. Comp. 2019, 55, 78-89
DOI: 10.1007/s10593-019-02422-8
65. Lichtenegger, M., Tiapko, O., Svobodova, B., Stockner, T., Glasnov, T.N., Schreibmayer, W., Platzer, D., de la Cruz, G.G., Krenn, S., Schober, R., Shrestha, N., Schindl, R., Romanin, C., Groschner, K.
An optically controlled probe identifies lipid-gating fenestrations within the TRPC3 channel
Nat. Chem. Biol. 2018, 14, 396-404
DOI: 10.1038/s41589-018-0015-6
64. Glasnov, T.
Photochemical Synthesis of Heterocycles: Merging Flow Processing and Metal-Catalyzed Visible Light Photoredox Transformations. In: Sharma U., Van der Eycken E. (eds) Flow Chemistry for the Synthesis of Heterocycles. Topics in Heterocyclic Chemistry, Vol 56. Springer, Cham. 2018
DOI: 10.1007/7081_2018_20
63. Bräuer, S., Borovicka, J, Glasnov, T., de la Cruz, G.G., Jensen, K.B., Gössler, W.
Homoarsenocholine - a novel arsenic compound detected for the first time in nature
Talanta 2018, 188, 107-110
DOI: 10.1016/j.talanta.2018.05.065
62. Schwendt, G. Glasnov, T.
Intensified synthesis of [3,4-d]triazole-fused chromenes, coumarins, and quinolones
Monatsh. Chem. 2017, 148, 69-75
DOI: 10.1007/s00706-016-1885-5
61. de la Cruz, G.G., Svobodova, B., Lichtenegger, M., Tiapko, O., Groschner, K., Glasnov, T.
Intensified microwave-assisted N-acylation procedure – Synthesis and activity evaluation of TRPC3 channel agonists with a 1,3-dihydro-2H-benzo[d]imidazol-2-one core
Synlett 2017, 28, 695-700
60. Tiapko, O., Bacsa, B., de la Cruz, G.G, Glasnov, T., Groschner, K.
Optopharmacological control of TRPC channels by coumarin-caged lipids is associated with a phototoxic membrane effect
Sci. China Life Sci. 2016, 59, 802-810
DOI: 10.1007/s11427-016-5095-5
59. Reichart, B., de la Cruz, G. G., Zangger, K., Kappe, C.O., Glasnov, T.
Copper/Nafion-catalyzed hydroarylation process involving ketenimine intermediates: A novel synthetic approach to 4-sulfonamidoquinoline-2-ones and derivatives thereof
Adv. Synth. Catal. 2016, 358, 50-55
58. Primessing, U., Schönleitner, P., Höll, A., Pfeiffer, S., Bracic, T., Rau, T., Kapl, M., Stojakovic, T., Glasnov, T., Leinweber, K., Wakula, P., Antoons, G., Pieske, B., Heinzel, F.R.
Novel pathomechanisms of cardiomyocyte dysfunction in a model of heart failure with preserved ejection fraction
Eur. J. Heart Fail. 2016, 18, 987-997
DOI: 10.1002/ejhf.524
57. Nussold, C., Üllen, A., Kogelnik, N., Bernhart, E., Reicher, H., Plasitra, I., Glasnov, T., Zangger, K., Rechberger, G., Kollroser, M., Fauler, G., Wolinski, H., Weksler, B.B., Romero, I.A., Kohlwein, S.D., Couraud, P.O., Malle, E., Sattler, W.
Assessment of electrophile damage in a human brain endothelial cell line utilizing a clickable alkyne analog of 2-chlorohexadecanal
Free Radic. Biol. Med. 2016, 90, 59-74
DOI: 10.1016/j.freeradbiomed.2015.11.010
56. Martinez, S.T., Belouezzane, C., Pinto, A.C, Glasnov, T.N.
Synthetic Strategies towards the Azabicyclo[3.3.0]-Octane Core of Natural Pyrrolizidine Alkaloids. An Overview review
Org. Prep. Proced. Int. 2016, 48, 223-253
DOI: 10.1080/00304948.2016.1165058
55. Glasnov, T.
Continuous-Flow Chemistry in the Research Laboratory - Modern Organic Chemistry in Dedicated Reactors at the Dawn of the 21st Century, 2016. Springer Int.
DOI: 10.1007/978-3-319-32196-7
2005-2015
54. Doleschal, B., Primessing, U., Wölkart, G., Wolf, S., Schernthaler, M., Lichtenegger, M., Glasnov, T.N., Kappe, C.O., Mayer, B., Antoons, G., Heinzel, F., Poteser, M., Groschner, K.
Cardiovasc. Res. 2015, 106, 163-173
DOI: 10.1093/cvr/cvv022
53. Hayden S., Glasnov, T.N., Kappe, C.O.
Chem. Eng. & Technol. 2015, 38, 1743-1748
52. Pieber, B., Glasnov, T., Kappe, C.O.
Chem. Eur. J. 2015, 21, 4368-4376
51. Üllen, A., Nussold, C., Glasnov, T., Saf, R., Cantillo, D., Eibinger, G., Reicher, H., Fauler, G., Bernhart, E., Hallstrom, S., Kogelnik, N., Zangger, K., Kappe, C.O., Malle, E., Sattler, W.
Biochem. Pharmacol. 2015, 93, 470-481
DOI: 10.1016/j.bcp.2014.12.017
50. Fekete, M, Glasnov, T. in Flow Chemistry. Fundamentals 2014. Berlin, Boston: De Gruyter.
ISBN 978-3-11-028916-9
49. Angelova, V.T., Frey, W., Ivanov, I.C., Vassilev, N., Glasnov, T.N.
Bulg. Chem. Commun. 2014, 46, 53-58
48. Dragoni, S., Turin, I., Laforenza, U., Potenza, D.M., Bottino, C., Glasnov, T.N., Prestia, M., Ferulli, F., Saitta, A., Mosca, A., Guerra, G., Rosti, V., Luinetti, O., Ganini, C., Porta, C., Pederazzoli, P., Tanzi, F., Montagna, D., Moccia, F.
Biomed Res. Internat. 2014, 23 p.
DOI: 10.1155/2014/739494
47. Gutmann, B., Elsner, P., Glasnov, T., Roberge, D.M., Kappe, C.O.
Angew. Chem. Int. Ed. 2014, 53, 11557-11561
46. Ivanov, I.C., Angelova, V.T., Tiritiris, I., Glasnov, T.
J. Heterocycl. Chem. 2014, 51, 1031-1035
DOI: 10.1002/jhet.2103
45. Lopes, R.O., de Miranda, A.S., Reichert, B., Glasnov, T., Kappe, C.O., Simon, R.C., Kroutil, W., Miranda, L.S.M., Leal, I.C.R., Souza, R.O.M.A.
J. Mol. Catal. B: Enzym. 2014, 104, 101-107
DOI: 10.1016/j.molcatb.2014.03.017
44. Martinez, S.T., Pinto, A.C., Glasnov, T., Kappe, C.O.
Tetrahedron Lett. 2014, 55, 4181-4184
DOI: 10.1016/j.tetlet.2014.05.055
43. Moghaddam, M.M., Pieber, B., Glasnov, T., Kappe, C.O.
ChemSusChem 2014, 7, 3122-3131
42. Pieber, B., Glasnov, T., Kappe, C.O.
RSC Adv. 2014, 4, 13430-13433
DOI: 10.1039/C4RA01442A
41. Ronco, V., Grolla, A.A., Glasnov, T.N., Canonico, P.L., Verkhratsky, A., Genazzani, A.A., Lim, D.
Cell Calc. 2014, 4, 219-229
DOI: 10.1016/j.ceca.2014.02.016
40. Baghbanzadeh, M., Glasnov, T., Kappe,C.O.
J. Flow Chem. 2013, 3, 109-113
39. Dalla-Vechia, L., Reichart, B., Glasnov, T., Miranda, L.S.M, kappe, C.O., Souza, R.O.M.A.
Org. Biomol. Chem. 2013, 11, 6806-6813
DOI: 10.1039/C3OB41464G
38. König, S, Schernthaner, M., Mächler, H., Kappe, C.O., Glasnov, T.N., Höfler, G., Braune, M., Wittchow, E., Groschner, K.
J. Pharmacol. Exp. Ther. 2013, 344, 33-40
37. Reichart, B., Kappe, C.O., Glasnov, T.N.
Synlett 2013, 24, 2393-2396
36. de la Cruz, G.G., Groschner, K., Kappe, C.O., Glasnov, T.N.
Tetrahedron Lett. 2012, 53, 3731-3734
DOI: 10.1016/j.tetlet.2012.04.120
35. Glasnov, T.N., Holbrey, J.D., Kappe, C.O., Seddon, K.R., Yan, T.
Green Chem. 2012, 14, 3071-3076
DOI: 10.1039/C2gc36226k
34. Morschhäuser, R., Krull, M., Kayser, C., Boberski, C., Bierbaum, P.A., Glasnov, T.N., Kappe, C.O.
Green Proc. Synth. 2012, 1, 281-290
33. Schleifer, H., Doleschal, B., Lichtenegger, M., Oppenrieder, R., Derler, I., Frischauf, I., Glasnov, T., Kappe, C.O., Romanin, C., Groschner, K.
Br. J. Pharmacol. 2012, 167, 1712-1722
DOI: 10.1111/j.1476-5381.2012.02126.x
32. Glasnov, T.N., Kappe, C.O.
J. Heterocycl. Chem. 2011, 48, 11-30
DOI: 10.1002/Jhet.568
31. Glasnov, T.N., Kappe, C.O.
Chem. Eur. J. 2011, 17, 11956-11968
30. Gutmann, B, Glasnov, T.N., Razzaq, T., Gössler, W., Roberge, D.M., Kappe, C.O.
Beilstein J. Org. Chem. 2011, 7, 503-517
DOI: 10.3762/bjoc.7.59
29. Irfan, M., Glasnov, T.N., Kappe, C.O.
Org. Lett. 2011, 13, 984-987
DOI: 10.1021/ol102984h
28. Irfan, M., Glasnov, T.N., Kappe, C.O.
ChemSusChem 2011, 4, 300-316
27. Obermayer, D., Glasnov, T.N., Kappe, C.O.
J. Org. Chem. 2011, 76, 6657-6669
DOI: 10.1021/jo2009824
26. Oliveria, M., Procopio, A., Glasnov, T.N., Gössler, W., Kappe, C.O.
Austr. J. Chem. 2011, 64, 1522-1529
DOI: 10.1071/Ch11125
25. Poteser, M., Schleifer, H., Lichtenegger, M., Schernthaner, M., Stockner, T., Kappe, C.O., Glasnov, T.N., Romanin, C., Groschner, K.
Proc. Nat. Acad. Sci. 2011, 108, 10556-10561
24. Viviano, M., Glasnov, T.N., Reichart, B., Tekautz, G., Kappe, C.O.
Org. Proc. Res. Dev. 2011, 15, 858-870
DOI: 10.1021/op2001047
23. Bariwal, J.B., Ermolatev, D.S., Glasnov, T.N., van Hecke, K., Mentha, V.P., van Meervelt, L., Kappe, C.O., van der Eycken, E.V.
Org. Lett. 2010, 12, 2774-2777
DOI: 10.1021/ol1008729
22. Damm, M., Glasnov, T.N., Kappe, C.O.
Org. Proc. Res. Dev. 2010, 14, 215-224
DOI: 10.1021/Op900297e
21. Glasnov, T.N., Kappe, C.O.
Adv. Synth. Catal. 2010, 352, 3089-3097
20. Glasnov, T.N., Findenig, S., Kappe, C.O.
Chem. Eur. J. 2009, 15, 1001-1010
19. Glasnov, T.N., Groschner, K., Kappe, C.O.
ChemMedChem 2009, 4, 1816-1818
18. Glasnov, T.N., Kappe, C.O.
Org. Synth. 2009, 86, 252-261
17. Irfan, M., Fuchs, M., Glasnov, T.N., Kappe, C.O.
Chem. Eur. J. 2009, 15, 11608-11618
16. Irfan, M., Petricci, E., Glasnov, T.N., Taddei, M., Kappe, C.O.
Eur. J. Org. Chem. 2009, 1327-1334
15. Razzaq, T., Glasnov, T.N., Kappe, C.O.
Eur. J. Org. Chem. 2009, 1321-1325
14. Razzaq, T., Glasnov, T.N., Kappe, C.O.
Chem. Eng. Technol. 2009, 32, 1702-1716
13. Uray. G., Kelterer, A.M., Hashim, J., Glasnov, T.N., Kappe, C.O., Fabian, W.M.F.
J. Mol. Stuct. 2009, 929, 85-96
DOI: 10.1016/j.molstruc.2009.04.009
12. Chebanov, V.A., Saraev, V.E., Desenko, S.M., Chernenko, V.N., Knyazeva, I.V., Groth, U., Glasnov, T.N., Kappe, C.O.
J. Org. Chem. 2008, 73, 5110-5118
DOI: 10.1021/jo800825c
11. Glasnov, T.N., Ivanov, I.C.
Synth. Commun. 2008, 38, 1579-1588
DOI: 10.1080/00397910801929465
10. Glasnov, T.N., Tye, H., Kappe, C.O.
Tetrahedron 2008, 64, 2035-2041
DOI: 10.1016/j.tet.2007.12.056
9. Ivanov, I.C., Gasnov, T.N., Belaj, F.
J. Heterocycl. Chem. 2008, 45, 177-180
8. Kappe, C.O., Stadler, A., Dallinger, D., Stohmeier, G., Perez, R., Zbruyev, O.I., Stiasni, N., Walla, P., Gorobets, N., Yousefi, B., Mont, N., Desai, B., Lengar, A., Krascsenicsova, K., Garbacia, S., Khanetskyy, B., Glasnov, T.N., Kremsner, J.M., da Silva, A.G.
New Methodologies and Techniques for a Sustainable Organic Chemistry 2008, 246, 221-251
DOI: 10.1007/978-1-4020-6793-8_11
7. Mennecke, K., Cecillia, R., Glasnov, T.N., Gruhl, S., Vogt, C., Feldhoff, A., Vargas, M.A.L., Kappe, C.O., Kunz, U., Kirschning, A.
Adv. Synth. Catal. 2008, 350, 717-730
6. Glasnov, T.N., Kappe, C.O.
Macromol. Rapid Commun. 2007, 28, 395-410
5. Glasnov, T.N., Kappe, C.O.
QSAR Comb. Sci. 2007, 26, 1261-1265
4. Hashim, J., Glasnov, T.N., Kremsner, J.M, Kappe, C.O.
J. Org. Chem. 2006, 71, 1707-1710
DOI: 10.1021/jo052283p
3. Glasnov, T.N., Vugts, D.J., Koningstein, M.M., Desai, B., Fabian, W.F., Orru, R.V.A., Kappe, C.O.
QSAR & Comb. Sci. 2006, 25, 509-518
2. Ivanov, I.C., Glasnov, T.N., Heber, D.
J. Heterocycl. Chem. 2005, 42, 857-861
1. Glasnov, T.N., Stadlbauer, W., Kappe, C.O.
J. Org. Chem. 2005, 70, 3864-3870
DOI: 10.1021/jo050254
 Toma Glasnov
Toma GlasnovAssoz. Prof. Dr. rer. nat.
Mag. Pharm.
Institut für Chemie
Schubertstr. 1/4